Ethyl Bromide is known as 74-96-4, Monobromoethane, Bromic ether, ethyl bromide, Ethane, bromo-, 1-Bromoethane, Hydrobromic ether, Bromure d'ethyle, ethylbromide with Molecular Formula of C2H5Br and Molecular Weight of 108.9651
It is manufactured through mixture of hydrogen bromide, ethyl alcohol, sulfuric acid, phosphorus and bromine method or through reaction of ethane with sulfur trioxide and potassium bromide.
Available in colorless to yellow liquid form with ether like odor, it comes with Boiling Point of 38.2°C, Melting Point of -119°C, Density/Specific Gravity of 1.4612 @ 20°C/4°C, Heat of Combustion of -1.2844X10+9 J/kmol, Heat of Vaporization of 3.3377X10+7 J/kmol @ -118.45°C. Further, it has miscible solubility in chloroform, org solvents, alcohol and ether.
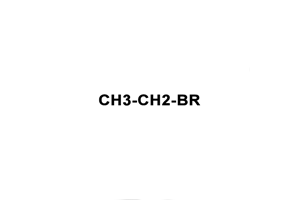
Ethyl Bromide